Antagonistic yeasts isolated from bioferments of mountain microorganisms (MM): an alternative for the control of fruit anthracnose caused by Colletotrichum tamarillo
Published 2025-12-30
Keywords
- Antagonistic yeasts,
- Colletotrichum,
- Anthracnose,
- Fruit rot,
- Mountain microorganisms
How to Cite
Copyright (c) 2025 Juan Alberto Trujillo-Salazar, Macela Mora Lopez, Daniel Andres Lopez Restrepo, Julian Esteban Pineda Montoya , Johana Patricia Ramírez-Olier , Liliana Rocío Botero-Botero

This work is licensed under a Creative Commons Attribution 4.0 International License.
Abstract
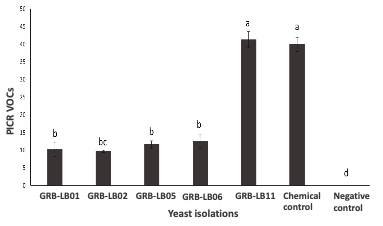
Antagonistic yeasts emerge as a friendly option for the control of anthracnose, a postharvest disease caused by phytopathogenic fungi of the genus Colletotrichum. This study evaluated, under laboratory conditions, the antagonistic activity of 24 native yeasts isolated from fermentations of mountain microorganisms (MM) against Colletotrichum tamarillo through dual confrontation tests, production of volatile organic compounds (VOCs) in mouth-to-mouth tests and the evaluation of the ability to control the development of rotting wounds infected with Colletotrichum tamarillo in tree tomato fruits (Solanum betaceum) in preventive and curative treatments. In this study, 5 of the 24 isolated yeasts showed antagonistic potential, Pichia kudriavzevii GRB-LB11 presented the highest antagonistic potential in dual culture (PICR of 41.3 ± 2.1%), and Pichia kudriavzevii GRB-LB02 presented the highest production of VOCs in mouth-to-mouth tests (67.3 ± 2.6%). For the tests on tree tomato fruits, Pichia kudriavzevii GRB-LB11 and Candida haemulonis GRB-LB06 controlled 100% of the incidence of the disease in the preventive treatments, reducing the diameters of the wounds caused by Colletotrichum tamarillo to 0 ± 0 cm. In the curative tests, Candida haemulonis GRB-LB06 managed to reduce the incidence of the disease by 96.8 ± 5.2, reducing the diameters of the wounds caused by Colletotrichum tamarillo to 0.1 ± 0.16. The results support the agroecological benefits of MM ferments and the biotechnological potential of isolated yeasts for anthracnose control
References
- Agrios, G. N. (1991). Fitopatología (1ra ed.). México: LIMUSA.
- Akwasi Akomeah Agyekum, George Soda Ayernor, Firibu Kwasi Saalia, & Betty Bediako-Amoa. (2015). Translocation of Pesticide Residues in Tomato, Mango, and Pineapple Fruits. Journal of Food Science and Engineering, 5(3). https://doi.org/10.17265/2159-5828/2015.03.006
- Arias Rivas, B., & Carrizales, L. (2007). Control químico de la antracnosis del mango (Mangifera indica l.) en pre y postcosecha en el Municipio Cedeño, Estado Monagas, Venezuela. Bioagro, 19(1), 19–25.
- Bautista-Rosales, P. U., Calderon-Santoyo, M., Servín-Villegas, R., Ochoa-Álvarez, N. A., & Ragazzo-Sánchez, J. A. (2013). Action mechanisms of the yeast Meyerozyma caribbica for the control of the phytopathogen Colletotrichum gloeosporioides in mangoes. Biological Control, 65(3), 293–+'301.
- Cabañas, C. M., Hernández, A., Martínez, A., Tejero, P., Vázquez-Hernández, M., Martín, A., & Ruiz-Moyano, S. (2020). Control of Penicillium glabrum by Indigenous Antagonistic Yeast from Vineyards. Foods, 9(12), 1864. https://doi.org/10.3390/foods9121864
- Cabrera, L., Rojas, P., Rojas, S., Pardo-De la Hoz, C. J., Mideros, M. F., Danies, G., … Restrepo, S. (2018). Most Colletotrichum species associated with tree tomato (Solanum betaceum ) and mango ( Mangifera indica ) crops are not host-specific. Plant Pathology, 67(5), 1022–1030.
- Caicedo, J. D., Lalangui, K. P., Pozo, A. N., Cevallos, P. A., Arahana, V. S., & Méndez, K. S. (2017). Multilocus molecular identification and phylogenetic analysis of Colletotrichum tamarilloi as the causal agent of Tamarillo (Solanum betaceum) anthracnose in the Ecuadorian highlands. European Journal of Plant Pathology, 148(4), 983–996.
- Campos-Martínez, A., Velázquez-del Valle, M. G., Flores-Moctezuma, H. E., Suárez-Rodríguez, R., Ramírez-Trujillo, J. A., & Hernández-Lauzardo, A. N. (2016). Antagonistic yeasts with potential to control Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. And Colletotrichum acutatum J.H. Simmonds on avocado fruits. Crop Protection, 89, 101–104. https://doi.org/10.1016/j.cropro.2016.07.001
- Chi, M., Li, G., Liu, Y., Liu, G., Li, M., Zhang, X., Sun, Z., Sui, Y., & Liu, J. (2015). Increase in antioxidant enzyme activity, stress tolerance and biocontrol efficacy of Pichia kudriavzevii with the transition from a yeast-like to biofilm morphology. Biological Control, 90, 113–119. https://doi.org/10.1016/j.biocontrol.2015.06.006
- Choque V., J., 2008. Producción de Humus de lombriz. La Paz: CIPCA. p 24 Diversidad Microbiana y Taxonomía, s/f. Consultado 5 diciembre 2012. Disponible en http://www.diversidadmicrobiana.com/index.php?option=com_content&view=article& id=671&Itemid=79
- Corbu, V., & Portocalelor, A. (2020). Biodiversity studies on Pichia kudriavzevii from romanian spontaneous fermented products. AgroLife Scientific Journal, 9(1).
- D.B. Wilson. Microbial diversity of cellulose hydrolysis. Curr Opin Microbiol, 14 (2011), pp. 259-263.
- De la Cruz-Quiroz, R., Roussos, S., Rodríguez-Herrera, R., Hernandez-Castillo, D., & Aguilar, C. N. (2018). Growth inhibition of Colletotrichum gloeosporioides and Phytophthora capsici by native Mexican Trichoderma strains. Karbala International Journal of Modern Science, 4(2), 237–243. https://doi.org/10.1016/j.kijoms.2018.03.002
- Fan, Y. V., Lee, C. T., Klemeš, J. J., Chua, L. S., Sarmidi, M. R., & Leow, C. W. (2018). Evaluation of Effective Microorganisms on home scale organic waste composting. Journal of Environmental Management, 216, 41–48. https://doi.org/10.1016/j.jenvman.2017.04.019
- Guarnaccia, V., Groenewald, J. Z., Polizzi, G., & Crous, P. W. (2017). High species diversity in Colletotrichum associated with citrus diseases in Europe. Persoonia - Molecular Phylogeny and Evolution of Fungi, 39(1), 32–50.
- Guzñay D, C. (2016). Guía Agroecológica para una agricultura resiliente en la parte baja de la Subcuenca del río Daula. Retrieved from https://www.avsf.org/public/posts/2254/guia_agroecologica_agricultura_resiliente_ecuador_avsf_2017.pdf
- Habiba, Noreen, R., Ali, S. A., Hasan, K. A., Sultana, V., Ara, J., & Ehteshamul-Haque, S. (2019). Evaluation of biocontrol potential of epiphytic yeast against postharvest Penicillium digitatum rot of stored Kinnow fruit (Citrus reticulata) and their effect on its physiochemical properties. Postharvest Biology and Technology, 148, 38–48. https://doi.org/10.1016/j.postharvbio.2018.10.007
- Hart, R. D. (1985). Conceptos Básicos sobre Agroecosistemas. Centro Agonomico Tropical de Investigación y Enseñanza.
- Higa, T., & Par, J. F. (1994). Beneficial and Effective Microorganisms for a Sustainable Agriculture and Enviroment. 25.
- Hernandez-Montiel, L. G., Gutierrez-Perez, E. D., Murillo-Amador, B., Vero, S., Chiquito-Contreras, R. G., & Rincon-Enriquez, G. (2018). Mechanisms employed by Debaryomyces hansenii in biological control of anthracnose disease on papaya fruit. Postharvest Biology and Technology, 139, 31–37. https://doi.org/10.1016/j.postharvbio.2018.01.015.
- Hjeljord L, Tronsmo A. Trichoderma and Gliocladium in biological control: an overview. In: Trichoderma & Gliocladium: Enzymes, biological control and commercial applications. Harman GE, Kubice CP. (Eds). Volumen 2. p.131-151. Taylor & Francis. 1998.
- Ingram, D. S. & Joachim, I. (1971). The growth of Peronospora farinosa f.sp, betae and sugar beet callus tissues in dual culture. Journal of General Microbiology 69, 211-220.
- Joshi, R. (2018). A Review on Colletotrichum spp. Virulence mechanism against host plant defensive factors. Journal of Medicinal Plants Studies, 6(6), 64–67. https://doi.org/10.22271/plants.2018.v6.i6b.02
- López Julián E., Gallego Jorge L., A Vargas-Ruiz lejandra, Amny Liceth Peña-Mosquera1 & Arley David Zapata-Zapata2 & Idalia Jacqueline López-Sánchez1 & Liliana Rocio Botero-Botero1 Aspergillus tubingensis and Talaromyces islandicus Solubilize Rock Phosphate Under Saline and Fungicide Stress and Improve Zea mays Growth and Phosphorus Nutrition Journal of Soil Science and Plant Nutrition (2020) 20:2490–2501. https://doi.org/10.1007/s42729-020-00315-w /
- Liu, J., Sui, Y., Wisniewski, M., Droby, S., & Liu, Y. (2013). Review: Utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. International Journal of Food Microbiology, 167(2), 153–160. https://doi.org/10.1016/j.ijfoodmicro.2013.09.004
- Liu, X., Gao, Y., Yang, H., Li, L., Jiang, Y., Li, Y., & Zheng, J. (2020). Pichia kudriavzevii retards fungal decay by influencing the fungal community succession during cherry tomato fruit storage. Food Microbiology, 88, 103404. https://doi.org/10.1016/j.fm.2019.103404
- Oro, L., Feliziani, E., Ciani, M., Romanazzi, G., & Comitini, F. (2018). Volatile organic compounds from Wickerhamomyces anomalus, Metschnikowia pulcherrima and Saccharomyces cerevisiae inhibit growth of decay causing fungi and control postharvest diseases of strawberries. International Journal of Food Microbiology, 265, 18–22. https://doi.org/10.1016/j.ijfoodmicro.2017.10.027
- Pérez N. Manejo Ecológico de plagas. CEDAR: La Habana. Cuba. 2004. 296 pp.
- Pushpa, T. B., Sekaran, V., Basha, S. J. S., & Jegan, J. (2016). Investigation on Preparation, Characterization and Application of Effective Microorganisms (EM) Based Composts—An Ecofriendly Solution. Nature Environment and Pollution Technology, 15(1), 7.
- Ramírez-Olier, J., Trujillo-Salazar, J., Osorio-Echeverri, V., Jaramillo-Ciro, M., & Botero-Botero, L. (2019). In vitro antagonism of Trichoderma asperellum against Colletotrichum gloeosporioides, Curvularia lunata, and Fusarium oxysporum Antagonismo in vitro de Trichoderma asperellum contra Colletotrichum gloeosporioides, Curvularia lunata, y Fusarium oxysporum. Revista UIS Ingenierías, 18, 7.
- Rungjindamai, N. (2016). Isolation and evaluation of biocontrol agents in controlling anthracnose disease of mango in Thailand. Journal of Plant Protection Research, 56(3), 306–311. https://doi.org/10.1515/jppr-2016-0034
- Sharma, G., Maymon, M., & Freeman, S. (2017). Epidemiology, pathology and identification of Colletotrichum including a novel species associated with avocado (Persea americana) anthracnose in Israel. Scientific Reports, 7(1), 15839.
- Sharma, V., Salwan, R., Sharma, Prem. N., & Kanwar, S. S. (2017). Elucidation of biocontrol mechanisms of Trichoderma harzianum against different plant fungal pathogens: Universal yet host specific response. International Journal of Biological Macromolecules, 95, 72–79. https://doi.org/10.1016/j.ijbiomac.2016.11.042
- Sigstad, E. E., Schabes, F. I., & Tejerina, F. (2013). A calorimetric analysis of soil treated with effective microorganisms. Thermochimica Acta, 569, 139–143. https://doi.org/10.1016/j.tca.2013.07.007
- Sipiczki, M., & Tap, R. M. (2016). Candida vulturna pro tempore sp. nov., a dimorphic yeast species related to the Candida haemulonis species complex isolated from flowers and clinical sample. International Journal of Systematic and Evolutionary Microbiology, 66(10), 4009–4015. https://doi.org/10.1099/ijsem.0.001302
- Spadaro, D., & Droby, S. (2016). Unraveling the mechanisms used by antagonistic yeast to control postharvest pathogens on fruit. Acta Horticulturae, (1144), 63–70. https://doi.org/10.17660/ActaHortic.2016.1144.9
- Spadaro, Davide, & Droby, S. (2016). Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends in Food Science & Technology, 47, 39–49. https://doi.org/10.1016/j.tifs.2015.11.003
- van Uden, N., & Kolipinski, M. C. (1962). Torulopsis haemulonii nov. spec. a yeast from the Atlantic ocean. Antonie van Leeuwenhoek, 28(1), 78–80. https://doi.org/10.1007/BF02538724
- Ventura-Aguilar, R. I., Bautista-Baños, S., Flores-García, G., & Zavaleta-Avejar, L. (2018). Impact of chitosan based edible coatings functionalized with natural compounds on Colletotrichum fragariae development and the quality of strawberries. Food Chemistry, 262, 142–149.
- Wilfrido, Y. Y., Alfredo, V.-A. L., Alfredo, L.-G. O., Patricio, V.-E. G., Cristina, L.-V. I., & Eduardo, C.-T. S. (2016). Efectos de un compost enriquecido con microorganismos eficientes sobre la germinación de semillas recalcitrantes de Artocarpus altilis (Parkinson) Fosberg y Theobroma cacao L. Effects of enriched compost with efficient microorganisms on the germination of recalcitrant seeds of breadfruit (Parkinson) Fosberg and Theobroma cacao L. Journal of the Selva Andina Biosphere, 4(2), 9.
- Zazzerini, A., & Tosi, L. (1985). Antagonistic activity of fungi isolated from sclerotia of Sclerotinia sclerotiorum. Plant Pathology, 34(3), 415–421.
- Zeballos Heredia, M. F. (2017). Caracterización de microorganismos de montaña (MM) en biofertilizantes artesanales. Escuale Agricola Panamericana, Honduras.
- Zhou, Y., Li, W., Zeng, J., & Shao, Y. (2018). Mechanisms of action of the yeast Debaryomyces nepalensis for control of the pathogen Colletotrichum gloeosporioides in mango fruit. Biological Control, 123, 111–119. https://doi.org/10.1016/j.biocontrol.2018.05.014





